Available Sizes:
| Vascular | Emergency |
| 1.4 in x 1.4 in (3.5 x 3.5 cm) | 3 in x 2 in (8 x 5 cm) |
| 2 in x 2 in (5 x 5 cm) | 3 in x 3 in (8 x 8 cm) (NSN Code: 6510-72-057-0464) |
Hemostatic Dressing
USFDA Cleared, 510(k) K202830
NSN codified

The Axiostat Patch is a sterile, single use, non-absorbable hemostatic dressing composed of Chitosan, a well-known natural polysaccharide which has widely recognized hemostatic properties. Axiostat’s structure allows it to rapidly absorb and lock blood plasma during its hemostasis action. Axiostat is available in 40+ countries across the globe.

Quick Hemostasis

Adaptive to multiple sheath sizes
Axiostat stops bleeding during withdrawal of sheath of all sizes

Acts as a Barrier to Bacteria


Clinical Use
- Vascular Procedure Sites
- Sites involving percutaneous catheters, tubes and pins
- Lacerations and Abrasions
- Skin Surface Puncture sites
- Surgical Debridement sites

How to use Axiostat

- Open the pack and remove Axiostat Patch.
- Can be cut or folded as per the wound size
- Place Axiostat Patch such that it covers the entire bleeding site. Apply uniform pressure until hemostasis is achieved.
- Do not move Axiostat Patch after applying.
- Use another Axiostat Patch in case bleeding is not controlled or dressing is completely saturated with blood.
- Apply a secondary dressing to ensure Axiostat Patch remains in position.
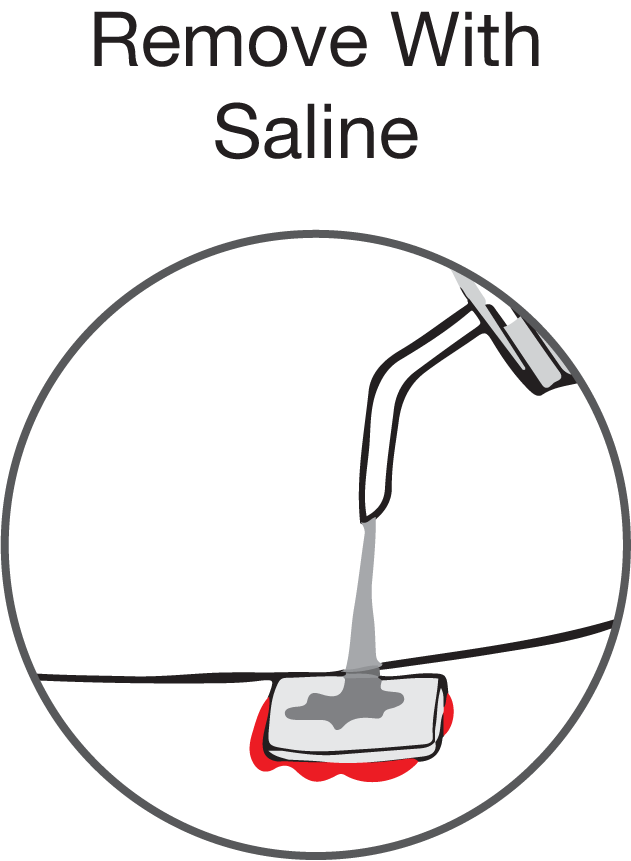
- Remove the Patch within 24 hours by irrigating with plenty of water or saline.

- Open the pack and remove Axiostat Patch.
- Can be cut or folded as per the wound size
- Place Axiostat Patch such that it covers the entire bleeding site. Apply uniform pressure until hemostasis is achieved.
- Do not move Axiostat Patch after applying.
- Use another Axiostat Patch in case bleeding is not controlled or dressing is completely saturated with blood.
- Apply a secondary dressing to ensure Axiostat Patch remains in position.

Remove the Patch within 24 hours by irrigating with plenty of water or saline.
Ordering Information – Vascular
| Product Description | Product Code | Product Size | Packaging |
| Axiostat® Patch V55 | V55 | 5 x 5 cm (2 x 2 in) | 10 Units |
| Axiostat® Patch V35 | V35 | 3.5 x 3.5 cm (1.4 x 1.4 in) | 10 Units |
Ordering Information – Emergency
| Product Description | NSN Code | Product Code | Product Size | Packaging |
| Axiostat® Patch L88 | 6510-72-057-0464 | L88 | 8 x 8 cm (3.15 x 3.15 in) | 10 Units |
| Axiostat® Patch M85 | — | M85 | 8 x 5 cm (3.15 x 2 in) | 10 Units |
The Axiostat Patch is a sterile, single use, non-absorbable hemostatic dressing composed of Chitosan, a well-known natural polysaccharide which has widely recognized hemostatic properties. Axiostat’s highly porous matrix with honeycomb microstructure with uniform porosity allows it to rapidly absorb and lock blood plasma during its hemostasis action. Axiostat is available in 50+ countries across the globe.
Composition:
Axiostat Patch is made up of chitosan extracted from shellfish. Natural material tends to change color. Hemostatic effectiveness remains un-changed.
Actions:
Indications:
Warnings:
- Remove within 24 hours.
- It is a non-absorbable hemostatic dressing. Do not implant.
- Do not use if patient is allergic to shellfish.
- Do not use if package is damaged or contents are wet.
- For single use only. Do not re-use or re-sterilize
- Axiostat Patch when used in vascular procedures, must be under the supervision of a trained healthcare professional. Observe sterile techniques at all times when using Axiostat Patch in vascular application. Recommend sterile gauze usage to apply uniform pressure and to hold Axiostat Patch in position during vascular procedures.
- After bleeding is controlled, do not remove Axiostat Patch immediately. Keep in position for at least 10 minutes.
- Axiostat Patch is intended for temporary control of bleeding only.
Precautions:
Notify physician if any of the following occur:
- Bleeding or significant drainage from the puncture site.
- Swelling, redness or warmth around the puncture site.
- Increased tenderness, numbness or cold sensation around the puncture site.
The safety and effectiveness of the Axiostat Patch has not been evaluated in the following patient populations:
- Patients with uncontrolled hypertension (>180 mm Hg systolic).
- Patients with bleeding disorders such as Thrombocytopenia (<100,000 platelet count), thrombasthenia, von Wille Brand’s disease, or anaemia (Hgb<10 mg/dl, Het<30).
- Patients receiving glycoprotein IIb/Illa inhibitors before, during, or after the catheterization procedure.
- Patients with bleeding diathesis or coagulopathy.
- Patients already having hematoma/pseudoaneurysm.
- Patients having ACT (Activated Clotting Time) > 180 sec.
- Patients undergoing therapeutic thrombolysis.
- Patients with auto-immune disorder and on medications for treating the same.
Refer the below tables for the duration of use of Axiostat Patch during vascular puncture procedures:
For Radial Access | |||
| Sheath Size | Heparin Dosage | Duration of manual compression with Axiostat Dressing | Time To remove secondary dressing |
|---|---|---|---|
| 6-8Fr Diagnostic Procedure | 5000 to 7000 units/mL | Approx. 3 minutes | Not before 45 minutes |
| 6-8Fr PCI(Percutaneous Coronary Intervention) | 7500 to 9000 units/mL | Approx. 5 minutes | Not before 90 minutes |
| 6-8Fr PCI(Percutaneous Coronary Intervention) or above | Above 9000 units/mL | Approx. 8 minutes | Not before 180 minutes |
For Femoral Access | |||
| Sheath Size | Heparin Dosage | Duration of manual compression with Axiostat Dressing | Time To remove secondary dressing |
|---|---|---|---|
| 6-8Fr Diagnostic Procedure | 5000 to 7000 units/mL | Approx. 5 minutes | Not before 360 minutes |
| 9-12Fr Diagnostic Procedure | 7500 to 12000 units/mL | Approx. 8 minutes | Not before 360 minutes |
| 6-12Fr PCI(Percutaneous Coronary Intervention) or above | 6000 to 12000 units/mL | Approx. 10 minutes | Not before 360 minutes |
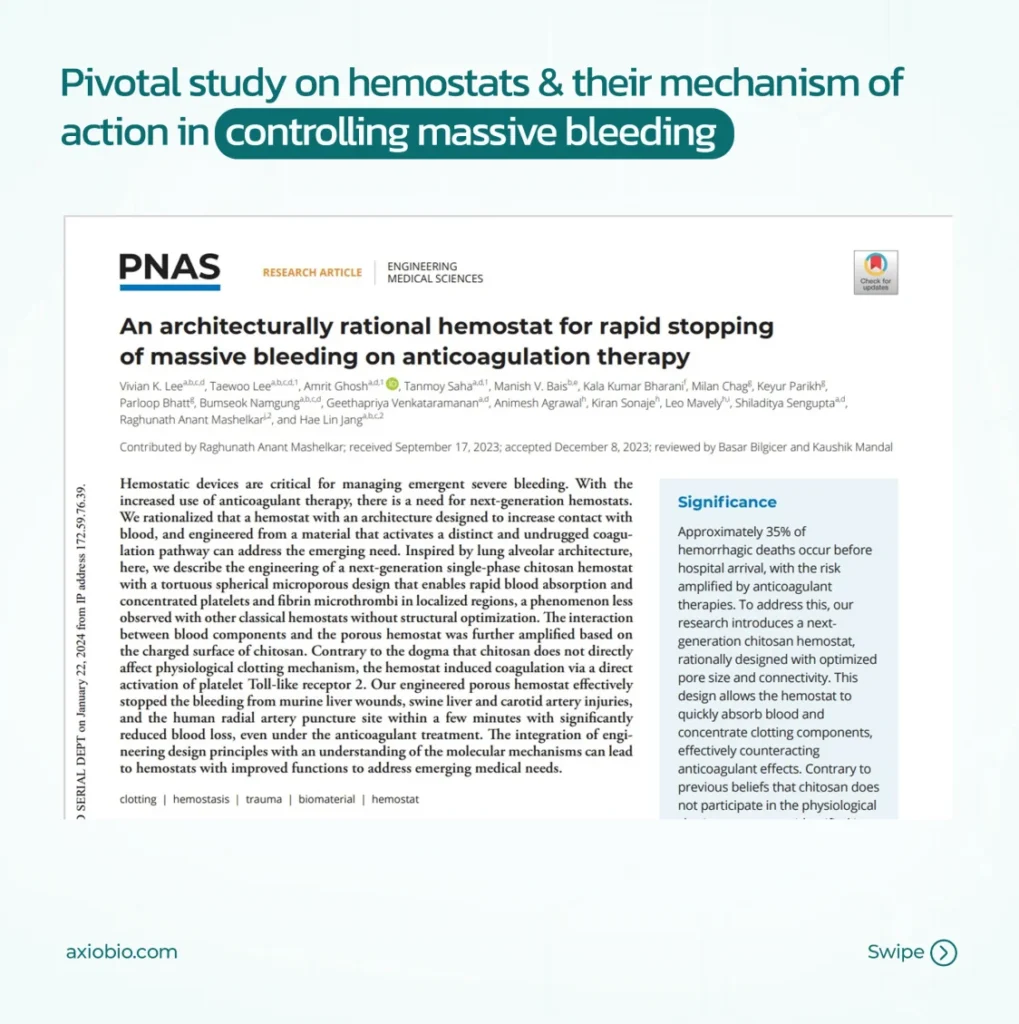
Pivotal study on Hemostats & their mechanism of action in controlling massive bleeding: A collaborative study with Harvard Medical School
Feel free to Contact us
To know more contact
office@advamedica.com
© Advamedica Inc. 2024, All Rights Reserved.